Published: November 12, 2023 | 5 mins read
Urine Supersaturation Impact on Kidney Stone Formation
Many factors contribute to kidney stone risk. One of these is urine supersaturation.
Supersaturation is the concentration of particles beyond the capacity of a liquid to hold in a solution. Think of this like the murky mixture of excess floating particles in your glass the last time you made a powdered drink mix like Cool-Aid and either used too little water or too much powdered mix.
Those leftover particles that did not dissolve mean the solution is supersaturated. In other words, nothing more can be dissolved.
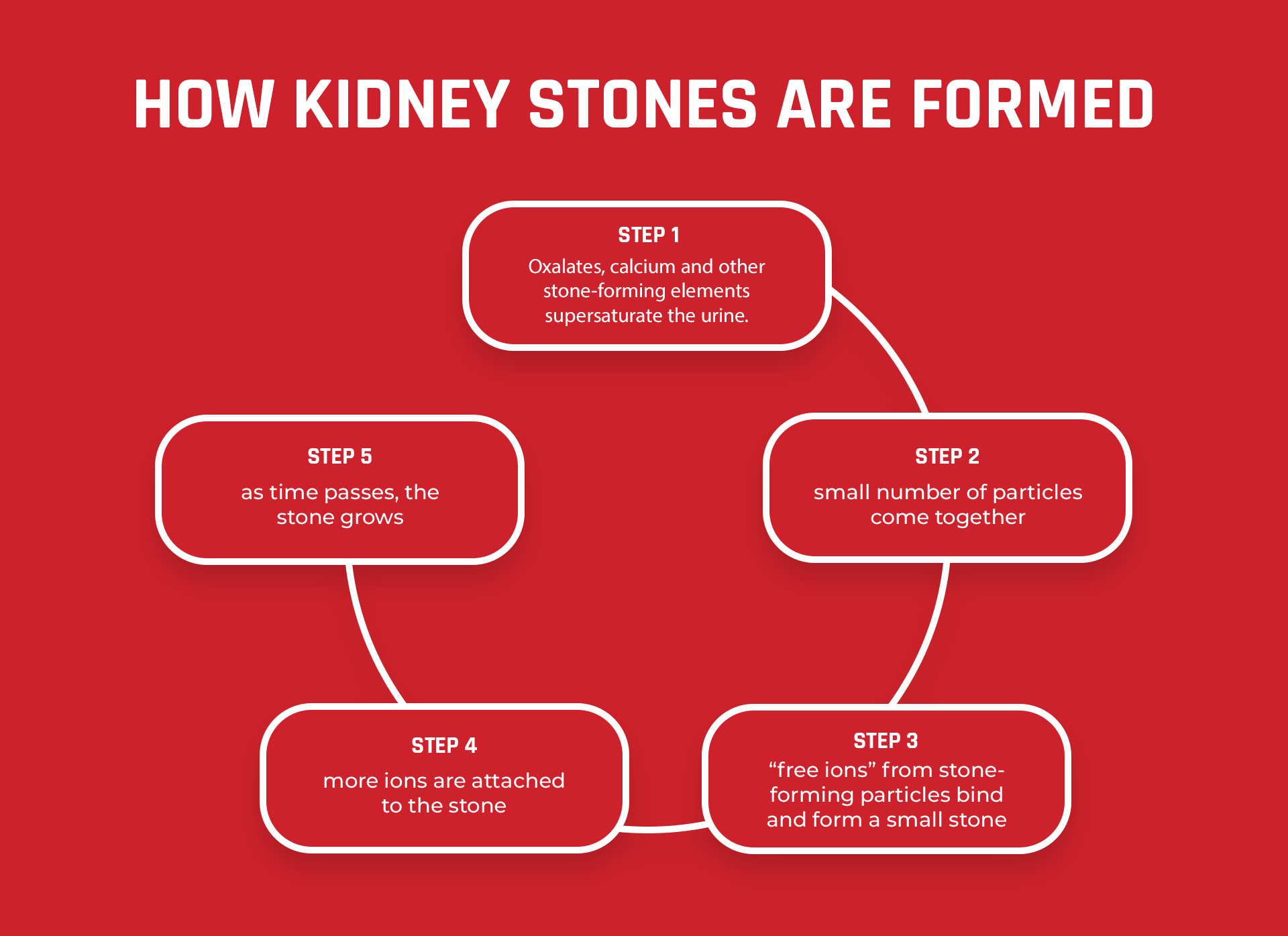
This same scenario happens in our kidneys when we have excess calcium, oxalate, and other materials floating around in our urine. Except when given time, they bind together to form kidney stones.
WHAT FACTORS AFFECT URINE SUPERSATURATION?
Many different elements are found in our urine. When these elements are left sitting in the kidneys, they may bind together and form stones.
Different stone types mean that certain elements are at higher concentrations than others. Outlined below are a few factors that affect supersaturation:
- The variety of elements present in the urine
- The total amount of these elements
- Urine volume
The most significant elements in kidney stone formation are calcium, oxalate, citrate, uric acid, sodium, magnesium, and phosphorus.
Urine pH is also essential because some stone types, like uric acid stones and calcium phosphate stones, are highly affected by urine acidity and alkalinity.
In the case of calcium-oxalate stone formers, the urine is supersaturated with calcium and oxalate. Of course, other substances can be present, but these two are the highest.
Additionally, inhibitors or elements with protective attributes against the stone formation, like citrate, are probably low. So is urine volume.
WHY DOES URINE SUPERSATURATION AFFECT STONE RISK?
The more time supersaturated elements such as calcium and oxalate sit in your kidneys, the higher your risk for kidney stones.
To explain this, let’s return to our example of the powdered drink mix from above. Since you didn’t use enough water (or used too much drink mix), you have excess particles still floating around in your glass.
Imagine that the glass is now your kidney. Instead of drink mix particles, it has elements such as calcium and oxalate.
Suppose you have excess calcium and oxalate (the drink mix) in your kidney (the glass). In that case, you either drink too little water or eat too many oxalate-containing foods.
If left unchecked, these excess elements of supersaturated calcium and oxalate (which won’t dissolve further) will start to bind to form kidney stones. This is how supersaturation impacts kidney stone risk.
HIGHER URINE SUPERSATURATION MEANS A HIGHER RISK
A computer program called EQUIL 2 is commonly used to measure supersaturation in urine samples. It computes the concentrations of free ions (from different elements) in the sample to get the Activity Product (AP) estimates.
The activity product (AP) is not a measurement unit, just numbers representing the degree of supersaturation of a substance in urine. It’s important to note that there are no “standard” values for urine supersaturation to be considered normal.
If you have a high supersaturation of calcium and oxalate, you are in danger of forming calcium oxalate stones. According to studies, if values (AP) are 1.0-1.9, you are already at risk.
In the same way, if your urine is highly supersaturated with calcium and phosphate, you might form calcium phosphate stones. Again, this is primarily true with all stone types. Highly alkaline urine is a risk factor for this stone type.
But, in reality, it is hard to determine precisely what elements supersaturate the urine using just a 24-hour urine collection. We eat different foods at different times of day and metabolize them at different rates. So, there’s no way to be sure that your urine tests will give you the most reliable result.

HOW TO PREVENT OR LOWER URINE SUPERSATURATION?
Kidney stone formation is directly connected to supersaturation. So the goal is to lower it. The foods we eat determine the variety of elements found in our urine. For instance, if you consume too many oxalate-rich foods, you will have more of these elements in your urine. This is why many people who suffer from calcium oxalate kidney stones go on a low-oxalate diet (<80mg/ day).
However, it’s important to note that oxalates are cumulative. That means, even if you eat low-oxalate foods only, you may still form kidney stones. So, if you are really looking for effective strategies to prevent kidney stones, we advise joining our Coaching Program. This is a real game-changer because you will have an opportunity to analyze your diet and lifestyle, plus we will help you formulate a personalized Prevention Plan just for you.
References
- How to use urine Supersaturations
- Supersaturation
- Kidney stones
- Relative Supersaturation of 24-Hour Urine and Likelihood of Kidney Stones
- Predictive Factors for Achieving the Recommended AUA Daily Urine Production in Patients With Nephrolithiasis
- EQUIL2: A Basic computer program for the calculation of urinary saturation
- Comparison of the Equil2 program and other methods for estimating the ion-activity product of urinary calcium oxalate: a new simplified method is proposed

Comments or questions?
Responses
WHAT TO READ NEXT
Publish Date: August 11, 2024
Magnesium Benefits for Kidney Stone-Formers
Publish Date: July 14, 2024
Medicines That Can Cause Kidney Stones
Publish Date: April 7, 2024
Metabolic Syndrome and Insulin Resistance Impact on Kidney Stones