Published: December 10, 2023 | 1 min read
You Can Stop Calcium Oxalate Stones If You Understand Randall’s Plaque
ARTICLE SHORTCUTS
- HOW PLAQUES FORM
- HOW KIDNEY STONES FORM IN RANDALL’S PLAQUE
- HOW TO PREVENT STONES FROM GROWING ON RANDALL’S PLAQUE
Before spilling the beans, let’s define Randall’s Plaque first.
A Randall’s Plaque appears to be an irregular, glossy, cream, or white-colored lesion in the kidney. Its size can range from 1-2mm up to covering the entire papilla (the tip of each renal pyramid).
Now, where did this lesion come from?
This is the cliché that never grows old- DIET. A Randall’s Plaque is made of calcium (carbonated in the urine) and phosphate. The sources of these two particles are mostly the foods we eat.
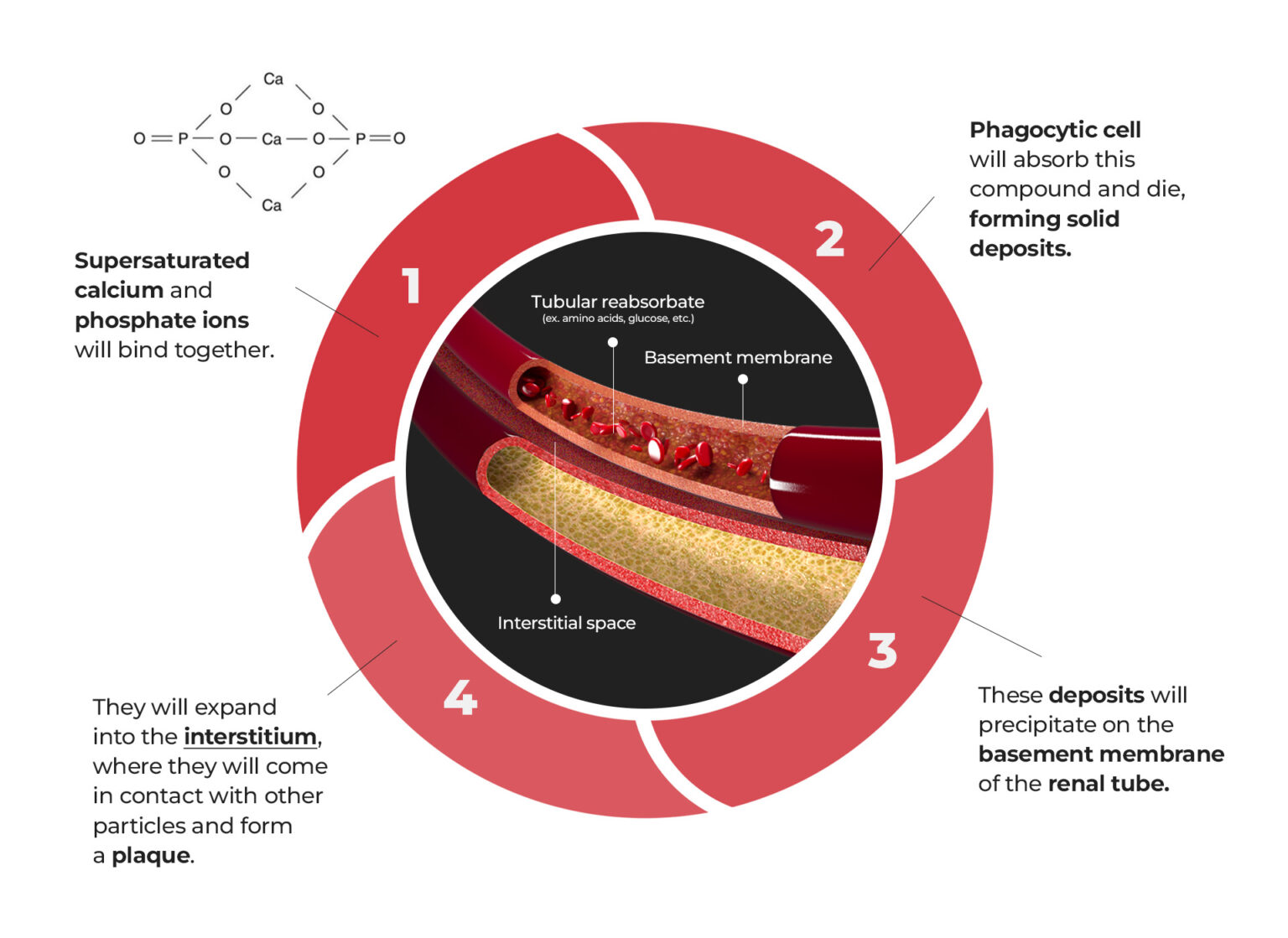
HOW PLAQUES FORM
When calcium and phosphate supersaturate the urine, they will bind together. Supersaturation means there are excess particles that the liquid can no longer dissolve.
A Phagocytic cell, which digests foreign particles, would then absorb this compound and die, forming solid deposits. These deposits will precipitate on the walls of the renal tubules (the basic unit of the kidney’s functional part) near the area where it empties water into the collecting ducts (see photo below).
After some time, they will expand into the interstitium or the spaces between the tubules and the vessels as it grows larger. This process is aided by collagen and carbohydrate molecules (mucopolysaccharide). Then, it will come in contact with other particles in the urine, thus forming plaques.
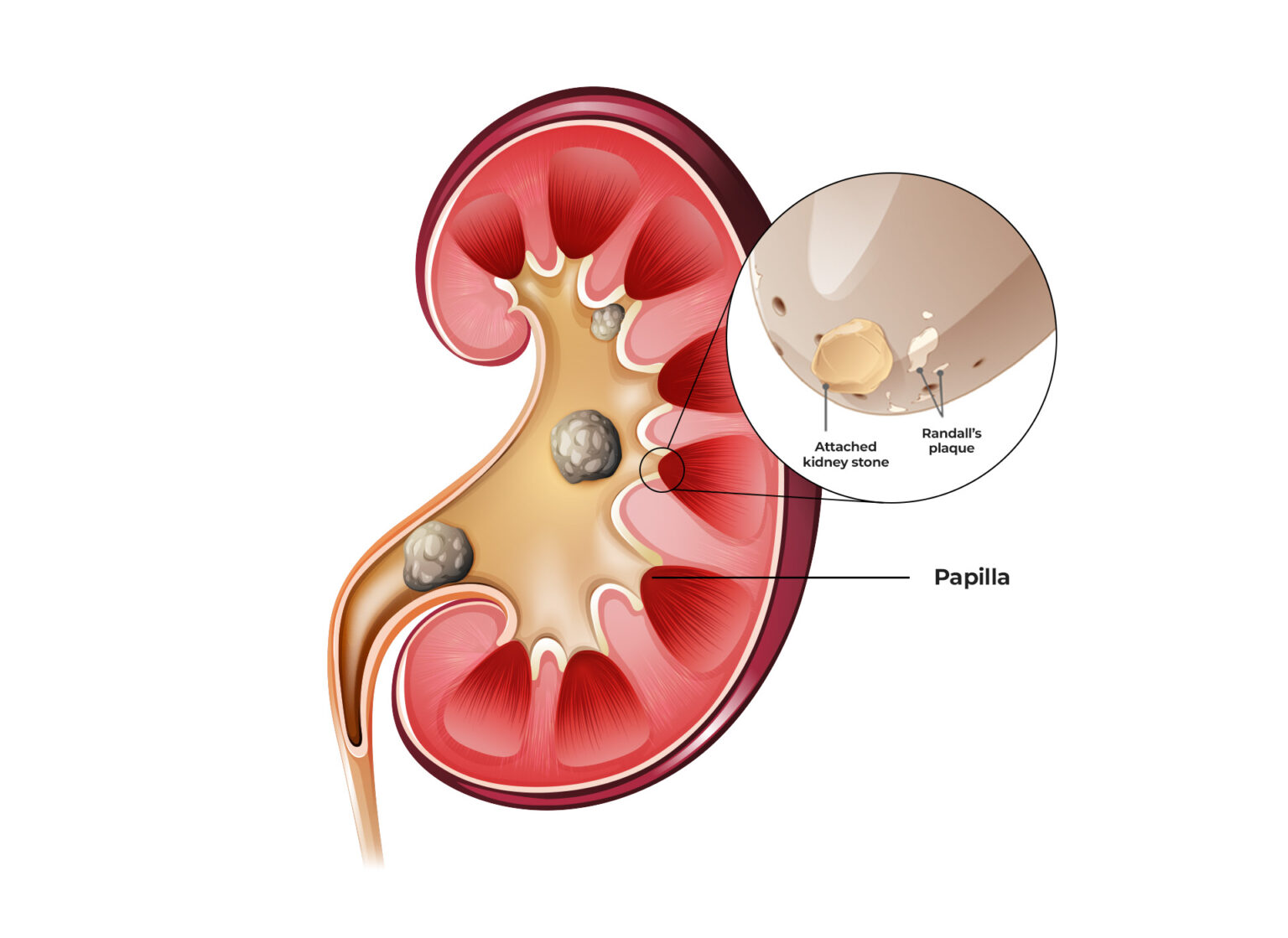
HOW KIDNEY STONES FORM IN RANDALL’S PLAQUE
Randall’s Plaque is basically harmless until exposed to elements in the urine. This exposure is the starting point of kidney stone formation. Crystals, especially calcium-based ones, will stick to the plaque, growing larger as more salts attract to it. The type of stone formed depends on the elements present in the urine.
So, the more it gets bathed in supersaturated urine (means there are undissolved particles), the more particles stick together, and the larger the stone will be.
Here’s a little secret: Randall’s Plaque is where calcium-oxalate stones originate. It’s ground zero for this stone type.
The process looks like this: calcium-phosphate crystals will get attracted to Randall’s Plaque (also made of calcium and phosphate). Then calcium-oxalate crystals will stick to it (because you munched on a spinach salad last night and had a large fries from McDonald’s for lunch).
HOW TO PREVENT STONES FROM GROWING ON RANDALL’S PLAQUE
Again, Randall’s Plaque is a toothless dragon. So it’s not really dangerous until it grows teeth – which will depend on you.
Let’s recap briefly: Randall’s Plaque is made of calcium and phosphate.
So, to troubleshoot plaque growth, we must control the supersaturation (excess free ions) of calcium and phosphate.
Should you limit calcium and phosphate? Nope. Those two are essential for your body. What you must do is boost your citrate intake.
The reason is that citrate ions also bind with calcium ions. Thus, it will prevent both phosphate and oxalates from binding with calcium.
For calcium stone formers, it is best to maintain 24-hour urinary calcium of <200 mg or <100 mg/L urine, while 24-hour urinary citrate should be around 320 mg/L urine.
Also, to ensure you aren’t putting yourself at risk of calcium oxalate stones, eliminate oxalates in your diet. Oxalates are 15 to 20 times stronger than calcium as a chemical promoter of kidney stones.
Urinary oxalate is considered “normal” up to about 40 mg/day. BUT the ultimate goal is to lower it to less than 25 mg/day (15-20 mg or less per liter of urine).
Crystal retention = stones
Basically, none of these crystals will form plaques (or ultimately stones) if you flush them out sooner because of proper hydration practices. The less you urinate, the longer these particles stay in your kidneys, and the better chances you develop nasty kidney stones.
REFERENCES
- 24-Hour Urine Testing for Nephrolithiasis: Interpretation Guideline
- Randall’s plaque as the origin of idiopathic calcium oxalate stone formation: an update
- Randall’s plaque and kidney stones: Recent advances and future challenges
- Randall’s Plaque
- TREATMENT OF IDIOPATHIC CALCIUM STONES
- The role of Randall plaques on kidney stone formation
Comments or questions?
Responses
You must be logged in to post a comment.

The core is really about avoiding oxalates ????