Published: February 18, 2024 | 7 mins read
Mixed Calcium Oxalate Kidney Stones: Subtypes and Causes
Certain kidney stones made of Calcium Oxalate can be a combination of Calcium-Oxalate Monohydrate (COM) and Calcium-Oxalate Dihydrate (COD). These mixed stones are almost as common as pure Calcium Oxalate stones. They make up 20.4% of all Calcium Oxalate stones, while “pure” COM or COD kidney stones are about 29.4% of the cases.
A “pure” stone means that a kidney stone contains at least 70% of a single mineral of either COM or COD. So, if a stone has less than 70% of a single mineral component, it is classified as mixed.
Notably, the percentage of COM versus COD molecules in these stones can vary due to specific reasons we will discuss in the next sections.
WHAT CAUSES MIXED COM AND COD STONES?
Mixed COM and COD stones form when you have these two main ingredients to crystallize their compositions.
- Hyperoxaluria
Hyperoxaluria means that there are high levels of oxalates in the urine. The primary source of oxalates is diet. That’s because oxalates are natural chemicals found in almost all plants. When you consume plant-based foods, these oxalates build up in your body, especially in the kidneys.
People prone to kidney stones should avoid eating plant-based foods (except ripe fruits), as oxalates are responsible for about 80% of kidney stone cases worldwide.
To prevent kidney stones, it’s recommended to keep your daily oxalate intake below 80mg to ensure that the amount of oxalates in your urine doesn’t exceed 25mg in 24 hours. However, due to the cumulative affect of oxalate, sometimes any amount of oxalate can be problematic.
If you want to know more about hyperoxaluria, read our Hyperoxaluria blog.
- Hypercalciuria
COD stones tend to form in urine with high calcium levels, a condition known as hypercalciuria. It means urinary calcium is more than 250mg in a 24-hour urine sample.
Many doctors would blame calcium intake for hypercalciuria. However, this is a total misconception. Calcium intake is not the real reason why urinary calcium increases. In fact, when you have hypercalciuria, you must meet the recommended daily allowance (RDA) for calcium, which is 1,200mg. Meeting the RDA for calcium is critical because you must replace the overly excreted calcium in your urine to maintain healthy levels in the body.
Here are the real culprits for high urinary calcium levels:
- High levels of Vitamin D
Vitamin D receptors transport calcium throughout the body. So, calcium transport will increase when a person has vitamin D sensitivity or has high vitamin D levels in the blood. This leads to higher calcium levels in the urine.
- Hyperparathyroidism
This condition occurs when the parathyroid glands (pea-sized glands in the neck) release too much parathyroid hormones (PTH). PTH helps regulate calcium levels in the blood, so when PTH levels are excessively high, it disrupts the body’s calcium balance.
For more in-depth information about hypercalciuria, read our Hypercalciuria blog.
With both hyperoxaluria and hypercalciuria, COD crystals will begin to form in the urine. Since the process of stone formation is not constant but occurs intermittently (on and off), COD crystals can partly change into COM.
But how does this transition take place exactly?
HOW DO COM AND COD CRYSTALS MIX?
A study on 1,310 mixed COM and COD stones showed that COD fragments can change into COM under specific conditions.
First, researchers found that they can make pure COD crystals in a water solution at temperatures between 5°C and 10°C. However, these COD crystals can completely transform into COM in periods ranging from 5 to 75 hours when temperature increases to 20°C up to 37°C. That’s because, at a higher temperature, COD crystals lose a water molecule from its structure. That means COD can lose a water molecule and become COM in a normal body temperature (37°C) and condition.
However, some COD crystals can remain unchanged. That’s when the solution has low-molecular-weight mineral constituents (<5000). On the other hand, COM always forms when high-molecular-weight mineral components (> 5000) are present. Nevertheless, COM stones contain only about 1.39% foreign elements, compared to COD stones with about 2.45% foreign elements. This suggests that foreign elements in the urine are mixed into the crystal structure of COD stones, which helps stabilize molecular arrangement.
THE 5 SUBTYPES OF MIXED COM AND COD STONES
Like COM and COD stones, there are subtypes of mixed Calcium-Oxalate stones. Listed below are the five known subtypes.
TYPE IIA + TYPE Ia/IIb + TYPE IVa

This stone type is typically characterized as follows:
- Whitish to light yellow
- Rounded
- Rough and uneven due to the presence of small spikes
- Having an umbilication or dent on the surface
The umbilication indicates the spot where it was previously attached to a calcium-phosphate plaque, called Randall’s Plaque, that accumulated in the kidney. The leading causes of these stones are hyperoxaluria (high oxalate levels) and hypercalciuria (high calcium levels).
TYPE 1a/IIA + TYPE Ib/IIB + TYPE IVa/IIb
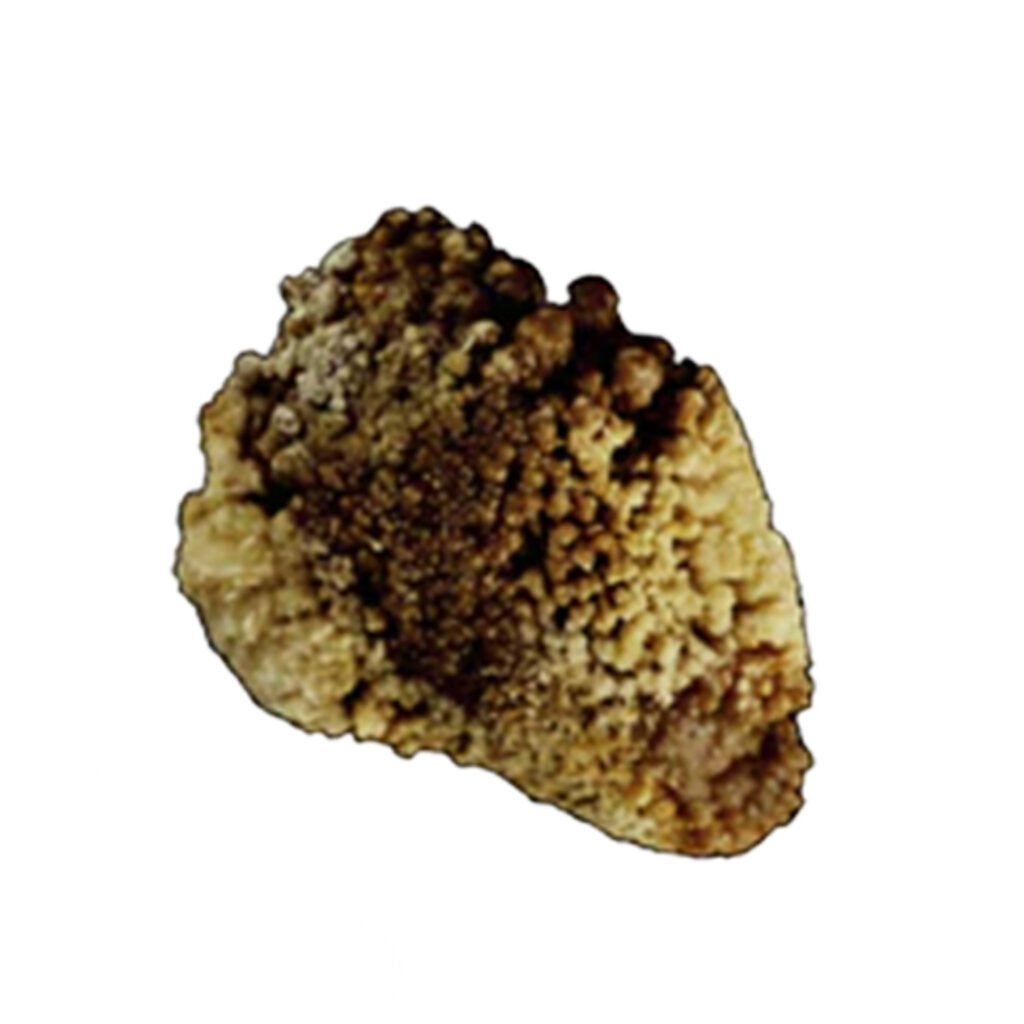
These stones have the following characteristics:
- Rough but less spiky surface
- Umbilication showing where they attached to Randall’s Plaque
- Yellowish-brown color, with some spots of dark brown
This irregularity in hue is likely a result of intermittent crystalline conversion from COD to COM. This phenomenon is impacted by moderate hypercalciuria, moderately high oxalate concentration, and low diuresis (urine output).
TYPE Ia/IIb + TYPE Ia + TYPE IVa

These stones are characterized by the following:
- Whitish color
- Appearing to have a few bumps and ridges
- Remarkable umbilication that is dark brown in color
Moderately high oxalate concentration, low diuresis, intermittent hypercalciuria, or Vitamin D hypersensitivity (toxicity or overdose) are the known causes of this type.
TYPE IIa/IVa1

These stones appear as follows:
- Having a rough surface
- Rounded shape
- Grayish color
These characteristics make them look like a sedimentary rock (a type of rock) in some cases. The primary cause of this type is hypercalciuria (high levels of urinary calcium).
TYPE IIa/IVa1

This stone type has the following characteristics:
- Yellowish or brownish color
- Some patches of white
- Irregular shape
- Rough surface
There are three possible reasons for this stone’s formation:
- Resorptive hypercalciuria: Increased calcium absorption in the gut associated with disorders or medications that enhance calcium absorption or excessive vitamin D intake.
- Absorptive hypercalciuria: Increased calcium excretion in the urine.
- Primary hyperparathyroidism: Overactivity of the parathyroid glands.
CONCLUSION
There are specific reasons why mixed stones of COM and COD form. And it’s easy to get lost in all these facts. However, if we look intently at the causes of why these stones form, we can draw one clear conclusion – you can prevent forming these stones through diet.
All these stones require two main things, “oxalates” and “calcium”. As explained previously, oxalates mainly come from the plant foods we eat. Shifting from plant-based to animal-based diets will prevent piling up oxalates in the body. In that way, no calcium oxalate stones will form even if your calcium levels are high.
Again, it’s not calcium intake that is the problem. You need a lot of calcium to maintain healthy bones, heart, cells, muscles, and nerves. For this reason, our best advice is to avoid oxalates and not calcium. Keep your calcium intake at 1,200mg to achieve optimal health.
If you want to explore more about the proper diet for kidney stones on a more personal level, feel free to check out our Coaching Program.

Comments or questions?
Responses
WHAT TO READ NEXT
Publish Date: August 18, 2024
Staghorn Kidney Stones Are BIG Problems!
Publish Date: August 4, 2024
Brushite Stones: The Most Complex Kidney Stones
Publish Date: July 28, 2024
The Deadly Struvite Stones